|
|
|

|
Thermochemistry
Copyright
D. Herrick |
 |
| |
20 Questions from past exams.
Practice for speed. Aim
for 2 minutes per problem.
|
|
| 1. |
Which
of the following are not state functions?
I-heat, II-work, III-energy,
IV-enthalpy
|
|
| A)
I |
| B)
I, II |
| C)
III, IV |
| D)
none |
| E)
all |
|
| 2. |
The
first law says the energy of a system is conserved if
the system is |
|
| A) |
at
constant temperature |
| B) |
at
constant volume |
| C) |
at
constant pressure |
| D) |
isolated
from the surroundings |
| E) |
thermally
insulated from the surroundings |
|
| 3. |
The
convention is that work done by the system is |
|
| A)
positive |
B)
zero |
C)
negative |
|
| 4. |
The
work (w) for a process that has DE
= 56 J and q = - 40 J is |
|
| A)
16 J |
B)
-96 J |
C)
zero |
D)
-16 J |
E) 96
J |
|
| 5. |
Given the following specific
heat
capacities of metals, which metal will reach the highest
temperature if the same amount of heat is added to 25 g
samples of each of the metals initially at 20.0ºC?
|
|
| A) |
aluminum
(MW = 26.98) |
0.900
J/g-ºC |
| B) |
calcium
(MW =40.08) |
0.653
J/g-ºC |
| C) |
copper
(MW = 63.55) |
0.385
J/g-ºC |
| D) |
gold
(MW = 197.0) |
0.129
J/g-ºC |
| E) |
nickel
(MW = 58.69) |
0.444
J/g-ºC |
|
| 6. |
Repeat
Question 5 for 1 mole samples of each metal. |
|
| A) |
aluminum |
D) |
gold |
| B) |
calcium |
E) |
nickel |
| C) |
copper |
|
|
|
| 7. |
The
first law says the energy change for a system is equal
to |
|
| A) |
the
heat absorbed by the system plus the work done by the
system |
| B) |
the
heat absorbed by the system plus the work done on the
system |
| C) |
the
heat emitted from the system plus the work done by the
system |
| D) |
the
heat emitted from the system plus the work done on the
system |
| E) |
none
of the above |
|
| 8. |
If
a chemical reaction at constant pressure gives off heat
to the surroundings the enthalpy change of the reaction
is |
|
| A)
positive |
B)
zero |
C)
negative |
|
| 9. |
The
reaction in Question 8 is called |
|
| A) |
endoergic |
| B) |
exoergic |
| C) |
endothermic |
| D) |
exothermic |
| E) |
hypothermic |
|
| 10. |
Calculate
the enthalpy change of 450 g of water as it cools from
90.0ºC to 30.0ºC. The heat capacity of water is
4.184 J/g-ºC. |
|
| A) |
27
kJ |
D) |
-6
kJ |
| B) |
113
kJ |
E) |
-113
kJ |
| C) |
6
kJ |
F) |
-27
kJ |
|
| 11. |
Given
these reactions 2 P(s) + 3 Cl2(g) 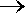 2
PCl3(l) , DHº
= -636 kJ 2
PCl3(l) , DHº
= -636 kJ
PCl5(s) 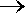 PCl3(l) + Cl2(g)
, DHº
= 137 kJ
PCl3(l) + Cl2(g)
, DHº
= 137 kJ
the standard heat of formation of solid PCl 5
is
|
|
| A) |
-499
kJ |
| B) |
-455
kJ |
| C) |
-568
kJ |
| D) |
-181
kJ |
| E) |
-
44 kJ |
|
| 12. |
Given
these gas reactions 2 HCl 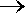 H2 + Cl2 , DHº
= 185 kJ H2 + Cl2 , DHº
= 185 kJ
2 H2 + O2 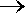 2 H2O , DHº
= -484 kJ
2 H2O , DHº
= -484 kJ
calculate DHº
for the following reaction:
H2O + Cl2 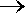 2 HCl + 0.5 O2
2 HCl + 0.5 O2
|
|
| A)
-299 kJ |
| B)
669 kJ |
| C)
427 kJ |
| D)
57 kJ |
| E)
none of the above |
|
| A) |
1324
kJ |
| B) |
1430
kJ |
| C) |
2434
kJ |
| D) |
732
kJ |
| E) |
626
kJ |
|
| 14. |
When
an endothermic reaction is carried out in a constant
pressure calorimeter the temperature of the surrounding
water bath is expected to |
|
| A)
increase |
| B)
decrease |
| A)
remain unchanged |
|
| 15. |
Use
the standard heats of formation
| Al2O3(s) |
-1676
kJ |
| HF(l) |
-300 kJ |
| H2O(l) |
-286 kJ |
| NaAlF4(s) |
-2017
kJ |
| NaClO3(s) |
-361
kJ |
| Na2CO3(s) |
-1131
kJ |
| Na2O(s) |
-417
kJ |
| SnCl2(s) |
-350
kJ |
to determine DHº
for the reaction
0.5 Na2O(s) + 0.5 Al2O3(s)
+ 4 HF(l)
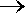 NaAlF4(s) + 2 H2O(l)
NaAlF4(s) + 2 H2O(l)
|
|
| A) |
-4696
kJ |
D) |
-4836
kJ |
| B) |
1217
kJ |
E) |
90
kJ |
| C) |
-156
kJ |
F) |
-342
kJ |
|
| 16. |
Given
the reaction A + 2 B 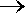 3 C, DHº
= 24 kJ
3 C, DHº
= 24 kJ
find DHº
for the reaction
3 C 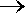 A + 2 B
A + 2 B |
|
| A) |
-12
kJ |
| B) |
-24
kJ |
| C) |
-36
kJ |
| D) |
-48
kJ |
| E) |
-64
kJ |
|
| 17. |
Calculate
the heat of formation of NO from the following heats of
reaction: 4NH3 + 5 O2
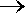 4 NO + 6 H2O , DHº
= -1170 kJ
4 NO + 6 H2O , DHº
= -1170 kJ
4NH3 + 3 O2 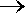 2 N2 + 6 H2O , DHº
= -1530 kJ
2 N2 + 6 H2O , DHº
= -1530 kJ
|
|
| A) |
-180
kJ |
D) |
90
kJ |
| B) |
180
kJ |
E) |
-360
kJ |
| C) |
-90
kJ |
F) |
360
kJ |
|
| 18. |
What's
the final temperature after a 20 kg bar of iron at 25ºC
absorbs 540 kJ of heat? The specific heat capacity
of iron is 0.450 J/g-ºC. |
|
| A) |
60ºC |
D) |
70ºC |
| B) |
75ºC |
E) |
80ºC |
| C) |
85ºC |
F) |
65ºC |
|
| 19. |
Given
the reaction A + 2 B 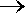 3 C, DHº
= 24 kJ
3 C, DHº
= 24 kJ
find DHº
for the production of 0.25 mole of C. |
|
| A) |
2
kJ |
D) |
96
kJ |
| B) |
4
kJ |
E) |
18
kJ |
| C) |
6
kJ |
F) |
72
kJ |
|
| 20. |
Given
the reactions A + 2 B 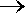 3 C, DHº
= 24 kJ
3 C, DHº
= 24 kJ
2 C 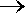 E,
DHº
= 12 kJ
E,
DHº
= 12 kJ
what is DHº
for the production of 24 moles of E from A and B. |
|
| A) |
504
kJ |
D) |
108
kJ |
| B) |
288
kJ |
E) |
672
kJ |
| C) |
84
kJ |
F) |
192
kJ |
|
|
|
|