|
|
|

|
Quantum Theory
Copyright
D. Herrick |
 |
| |
20 Questions from past exams.
Practice for speed. Aim
for 2 minutes per problem.
|
|
| |
Constants:
Avogadro's number:
NA = 6.02 × 1023
/ mole
speed of
light: c = 3.00 × 108
m/s
Planck's
constant: h = 6.63 × 10-34
J-s
electron volt: 1 eV =
1.60 × 10-19 J
calorie: 1 cal = 4.184 J
Angstrom: 1 Å = 1 × 10-10
m = 100 pm |
|
|
|
| 1. |
Which
wavelength of light has the highest frequency? |
|
| A) 1000Å |
B)
800nm |
C)
0.001cm |
D)
0.01mm |
|
| 2. |
Calculate
the frequency (s-1) of green light of
wavelength 5000Å |
|
| A) |
6.85
× 1015 |
| B) |
2.34
× 1015 |
| C) |
0.60
× 1015 |
| D) |
1.28
× 1015 |
| E) |
0.49
× 1015 |
|
|
| 3. |
The
energy (J) of one green photon of wavelength
5000Å is |
|
| A) |
2.14
× 10-19 |
| B) |
3.36
× 10-19 |
| C) |
9.98
× 10-18 |
| D) |
1.67
× 10-19 |
| E) |
3.98
× 10-19 |
|
| 4. |
The
energy (kJ) of one mole of green photons of wavelength
5000Å is |
|
| A)
240 |
B)
132 |
C)
216 |
D)
306 |
E)
347 |
|
| 5. |
The
energy in J/photon of blue light at a wavelength of
424.0 nm is |
|
| A) |
6.28
× 10-11 |
| B) |
4.69
× 10-19 |
| C) |
7.07
× 10-20 |
| D) |
6.42
× 10-17 |
| E) |
2.81
× 10-38 |
|
| 6. |
According
to Planck the energy of a photon is |
|
| A) |
proportional
to the square of the radiation frequency |
| B) |
proportional
to the inverse of the wavelength |
| C) |
independent
of the color of the radiation |
| D) |
proportional
to the inverse square of the frequency |
| E) |
proportional
to the wavelength |
|
| 7. |
The
initial step in reactions that deplete atmospheric ozone
is photodissociation of a chlorofluorocarbon molecule at
a wavelength of 200 nm, CF2Cl2 + light
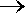 CF2Cl + Cl
CF2Cl + Cl
The energy of this radiation in kJ per mole of
photons is
|
|
| A) 178 |
B)
39 |
C)
854 |
D) 599 |
E)
361 |
|
| 8. |
Which
is NOT consistent with the Bohr theory? |
|
| A) |
The
classical picture of an electron moving in a circular orbit around the
nucleus is replaced by a standing wave for the electron. |
| B) |
The
energy of an electron is quantized. |
| C) |
An
electron may move to a lower orbit by emitting radiation
of frequency proportional to the energy difference
between the two orbits. |
| D) |
The
radius of an orbit increases at higher energy levels. |
| E) |
The
energy of an orbit is independent of the nuclear charge. |
|
| 9. |
The
Bohr radius of hydrogen in the ground state is 0.529Å.
The radius of a hydrogen atom with n = 7 is |
|
| A) 92.6Å |
B) 3.70Å |
C)
25.9Å |
D) 0.529Å |
E)
52.9Å |
|
| 10. |
The
Bohr radius of hydrogen in the ground state is 52.9pm.
The Bohr radius of a helium ion He+ in the
ground state is |
|
| A)
212pm |
B)
26.5pm |
C)
13.2pm |
E)
52.9pm |
E)
106pm |
|
| 11. |
The
radius of a Bohr orbit in terms of the quantum number n
and the nuclear charge Z is proportional to |
|
| A)
n 2 / Z |
B)
n2 / Z 2 |
C)
n / Z 2 |
D)
n / Z |
|
| 12. |
The
energy to ionize an electron from a Bohr orbit is
proportional to |
|
| A)
Z/n2 |
B)
Z2 / n |
C)
Z 2 / n 2 |
D)
Z / n |
|
| 13. |
According
to deBroglie the quantum wavelength of a particle of
mass m moving with velocity v is
|
|
| A) |
proportional
to (mv) |
| B) |
proportional
to the square of (mv) |
| C) |
independent
of (mv) |
| D) |
proportional
to the inverse of (mv) |
| E) |
proportional
to the inverse square of (mv) |
|
| 14. |
Which
is an allowed set of quantum numbers
(n, l, m) for the hydrogen atom? |
|
| A) |
(4,
-2, 1) |
| B) |
(24,
12, -14) |
| C) |
(8,
8, 8) |
| D) |
(213,
210, 198) |
| E) |
(11,
-5, -5) |
|
| 15. |
In
which orbital does the electron in a hydrogen atom have
the largest ionization energy? |
|
| A) |
3p |
D) |
9d |
| B) |
8d |
E) |
7p |
| C) |
2s |
F) |
15f |
|
| 16. |
In
which orbital does the electron in a hydrogen atom have
the largest angular momentum? |
|
| A) |
10s |
D) |
23p |
| B) |
8f |
E) |
34d |
| C) |
5d |
F) |
9p |
|
| 17. |
How
many spatial orbitals are there in the 5f subshell? |
|
| A) |
7 |
D) |
5 |
| B) |
25 |
E) |
15 |
| C) |
75 |
F) |
35 |
|
| 18. |
The
allowed values of the magnetic quantum number for
spatial orbitals with n = 3 in the H atom are |
|
| A) |
0,1,2 |
D) |
3,2,1,0,-1,-2,-3 |
| B) |
0,1,2,4 |
E) |
-1,0,1 |
| C) |
-2,-1,0,1,2 |
F) |
0,1,2,3 |
|
| 19. |
What
is the total number of spatial orbitals with principal
quantum number 4 in the C5+ ion? |
|
| A) 9 |
B) 25 |
C)
80 |
D) 49 |
E)
16 |
|
| 20. |
Light
emitted from the transition 3d 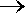 2p in the hydrogen atom has l
= 656 nm. What is the wavelength of light
from the transition 500f 2p in the hydrogen atom has l
= 656 nm. What is the wavelength of light
from the transition 500f 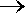 499d in hydrogen? 499d in hydrogen? |
|
| A) |
5680
nm |
F) |
5.68
mm |
| B) |
123
pm |
G) |
47.1
mm |
| C) |
47.1
cm |
H) |
568
cm |
| D) |
56.8
nm |
I) |
1.23
mm |
| E) |
12.3
m |
J) |
4.71
m |
|
|
|
|