
|
CONSTANTS |
HYDROGEN-LIKE
ATOMS |

STANDARD HEATS OF FORMATION
|
PERIODIC TABLE OF ELEMENTS
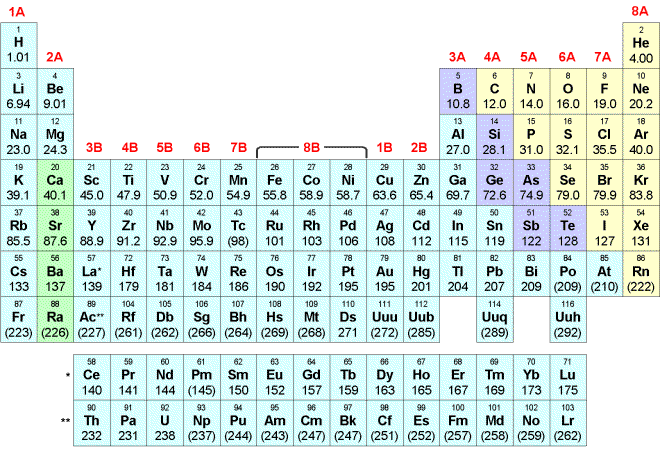
Select one answer for each question:
1. Which statement is TRUE?
| A) | As the energy increases, the frequency of the radiation decreases. | |
| B) | The product of wavelength and frequency of light is a constant. | |
| C) | As the wavelength of the light increases, the frequency increases. | |
| D) | Red light has a higher frequency than blue light. | |
| E) | Light is considered to have only wave character. | |
2. The electron configuration of Na is
| A) | 1s2 2s2 2p6 3p | |
| B) | 1s2 2s2 2p6 3s | |
| C) | 1s2 2s2 2p3 3s | |
| D) | 1s2 2s2 2p3 3d | |
| E) | 1s2 2s2 2p3 3p | |
3. When Ne is ionized the orbital from which the electron is removed has
| A) | n = 1, l = 0 | |
| B) | n = 1, l = 1 | |
| C) | n = 2, l = 2 | |
| D) | n = 2, l = 1 | |
| E) | n = 2, l = 0 | |
4. In
which atom does the electron configuration in the highest subshell look like
this?
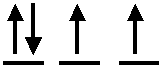
|
|||
| A) | Te | ||
| B) | As | ||
| C) | Sn | ||
| D) | Sb | ||
| E) | Cl | ||
5. How many orbitals are there in the n=4 level of the hydrogen atom?
| A) | 14 | |
| B) | 6 | |
| C) | 16 | |
| D) | 10 | |
| E) | 20 | |
6. How many electrons can be filled into the 5d subshell?
| A) | 10 | |
| B) | 25 | |
| C) | 15 | |
| D) | 5 | |
| E) | 12 | |
7. How many electrons in Xe occupy d orbitals with ml = +1?
| A) | 10 | |
| B) | 18 | |
| C) | 2 | |
| D) | 6 | |
| E) | 4 | |
8. Which of these atoms has the smallest radius?
| Size trends of atoms other than hydrogen will not be on the CH221 final | ||
| A) | Xe | |
| B) | Al | |
| C) | Ca | |
| D) | F | |
| E) | N | |
9. According to ____ a particle of mass m and velocity v has a wavelength l = h/mv.
| A) | Rutherford | |
| B) | de Broglie | |
| C) | Heisenberg | |
| D) | Bohr | |
| E) | Planck | |
10. Which shape is consistent with a 3p orbital?
| A) | B) | C) | D) | E) | |
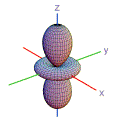 |
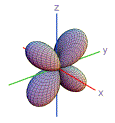 |
 |
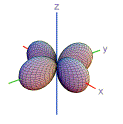 |
 |
11. "Paramagnetic" atoms are attracted to a magnetic field because they contain unpaired electron spins. Which atom is NOT paramagnetic?
| A) | Cs | |
| B) | P | |
| C) | Mg | |
| D) | Al | |
| E) | O | |
12. According to ________ the energy of a light wave in the photoelectric effect is the E = hn.
| A) | Einstein | |
| B) | Bohr | |
| C) | Heisenberg | |
| D) | Pauli | |
| E) | Davison and Germer | |
13. The best description of the atom reaction Na + Cl ® Na+ + Cl- is:
| A) | The electron jumps from the 3p orbital on Na to the 3p orbital on Cl. | |
| B) | The electron jumps from the 3s orbital on Na to the 3s orbital on Cl. | |
| C) | The electron jumps from the 3s orbital on Na to the 3p orbital on Cl. | |
| D) | The electron jumps from the 3p orbital on Na to the 3s orbital on Cl. | |
14. The ionization energy ("work function") of cesium is 6.24 ´ 10-19 J/atom. Find the minimum frequency of light required to ionize a cesium atom.
| A) | 1.06 ´ 10-15 s-1 | |
| B) | 9.42 ´ 1011 s-1 | |
| C) | 1.06 ´ 10-12 s-1 | |
| D) | 9.41 ´ 1014 s-1 | |
| E) | 4.13 ´ 1014 s-1 | |
15. What is the wavelength (in nanometers) of light emitted when the hydrogen atom undergoes a transition from level n = 5 to level n = 2?
| A) | 663 nm | |
| B) | 833 nm | |
| C) | 604 nm | |
| D) | 546 nm | |
| E) | 434 nm | |
16. What is the ionization energy (in kJ/mole) of the hydrogen-like ion F8+ ?
| A) | 1.06 ´ 103 | ||
| B) | 1.31 ´ 104 | ||
| C) | 1.59 ´ 105 | ||
| D) | 1.06 ´ 105 | ||
| E) | 8.40 ´ 104 | ||
17. The mass (in grams) of one N2O4 molecule is:
| A) | 92.0 | |
| B) | 46.0 | |
| C) | 1.53 ´ 10-22 | |
| D) | 1.15 ´ 10-22 | |
| E) | 7.64 ´ 10-23 | |
18. When HCl reacts with NaOH in aqueous solution the net ionic equation is
| A) | Na+(aq)
+ Cl-
(aq) |
|
| B) | H+(aq)
+ OH-(aq)
|
|
| C) | OH-(aq)
+ HCl(aq) |
|
| D) | H+(aq)
+ NaOH(aq) |
|
| E) | no reaction | |
19. What is the change in enthalpy (kJ) when 1 mole (28.0 g) of carbon monoxide is oxidized to carbon dioxide?
2 CO(g) + O2 (g) ![]() 2 CO2 (g) + 566 kJ
2 CO2 (g) + 566 kJ
| A) | -566/2 | |
| B) | -566 | |
| C) | 566 / 2 | |
| D) | 566 | |
| E) | 566 ´ 2 | |
20. Which of these ionic compounds is least soluble in water?
| A) | (NH4)2CO3 | |
| B) | Pb(NO3)2 | |
| C) | MgCl2 | |
| D) | Ca3(PO4)2 | |
| E) | NaCl | |
21. Oxidation-reduction reactions involve transfer of
| A) | electrons to the reducing agent from the oxidizing agent | |
| B) | protons to the oxidizing agent from the reducing agent | |
| C) | electrons to the oxidizing agent from the reducing agent | |
| D) | protons to the reducing agent from the oxidizing agent | |
22.
The oxidation number of antimony in SbCl63-
is
| A) | 5 | |
| B) | 3 | |
| C) | 6 | |
| D) | 4 | |
| E) | 2 | |
23. Balance the equation with lowest integer coefficients and give the coefficient of H+.
_Fe2+
+ _ H+ + _Cr2O72-
![]() _Cr3+ + _Fe3+ + _ H2O
_Cr3+ + _Fe3+ + _ H2O
| balancing redox equations will not be on the CH221 final | ||
| A) | 16 | |
| B) | 18 | |
| C) | 12 | |
| D) | 10 | |
| E) | 14 | |
24. The reactant that is reduced in the equation in Question 23 is:
| A) | Cr2O72- | |
| B) | H+ | |
| C) | Fe2+ | |
| D) | none--this is not a redox reaction | |
25. Use these two reactions
2 Fe + 1.5 O2 ![]() Fe2O3 ,
DH
= -823 kJ
Fe2O3 ,
DH
= -823 kJ
3 Fe + 2 O2 ![]() Fe3O4 ,
DH
= -1120 kJ
Fe3O4 ,
DH
= -1120 kJ
to find the enthalpy change for the reaction
3 Fe2O3
![]() 2 Fe3O4
+ 0.5 O2
2 Fe3O4
+ 0.5 O2
| A) | 256 kJ | |
| B) | -229 kJ | |
| C) | 245 kJ | |
| D) | 229 kJ | |
| E) | -256 kJ | |
26. A chemical system at constant pressure absorbs 50 kJ of heat from the surroundings. The enthalpy change of the system is best described as:
| A) | DH = -50 kJ (endothermic) | |
| B) | DH = 50 kJ (exothermic) | |
| C) | DH = 50 kJ (endothermic) | |
| D) | DH = -50 kJ (exothermic) | |
27. How much heat is lost when 35.5 g of iron cools from 429°C to 18.6°C ? The specific heat of iron is 0.450 J/g-°C.
| A) | 14,600 J | |
| B) | 32,400 J | |
| C) | 4820 J | |
| D) | 8240 J | |
| E) | 6560 J | |
28. Find the moles of electrons transferred in the balanced oxidation-reduction reaction
C6H12O6
+ 6 O2 ![]() 6 CO2 + 6 H2O
6 CO2 + 6 H2O
| this type of redox problem will not be on the CH221 final | ||
| A) | 16 | |
| B) | 24 | |
| C) | 48 | |
| D) | 36 | |
| E) | 12 | |
29. How many moles of atoms are there in one mole of iron (III) sulfate?
| A) | 18 | |
| B) | 16 | |
| C) | 15 | |
| D) | 17 | |
| E) | 20 | |
30. The empirical formula of a compound SbxOy that contains 75.31% antimony (Sb) by weight is
| A) | SbO2 | ||
| B) | Sb2O3 | ||
| C) | Sb2O5 | ||
| D) | SbO4 | ||
| E) | SbO3 | ||
31.
How many grams of sulfate ions are in 0.200 L of
0.0300 M Al2(SO4)3
solution?
| A) | 2.88 | |
| B) | 1.73 | |
| C) | 8.06 | |
| D) | 1.15 | |
| E) | 2.73 | |
32. How many mL of 0.080 M sulfuric acid (H2SO4, MW = 98.1) are required for complete neutralization of 40.0 mL of 0.050 M aluminum hydroxide (Al(OH)3, MW = 78.0)?
| A) | 37.5 | |
| B) | 16.7 | |
| C) | 51.4 | |
| D) | 62.8 | |
| E) | 75.1 | |
33. What is the theoretical mass yield of tetraphosphorous hex(a)oxide produced by combustion of 8.00 g of phosphorous in excess oxygen?
| A) | 42.6 g | ||
| B) | 227. g | ||
| C) | 56.8 g | ||
| D) | 28.4 g | ||
| E) | 14.2 g | ||
