
|
CONSTANTS |
HYDROGEN-LIKE
ATOMS |

STANDARD HEATS OF FORMATION
|
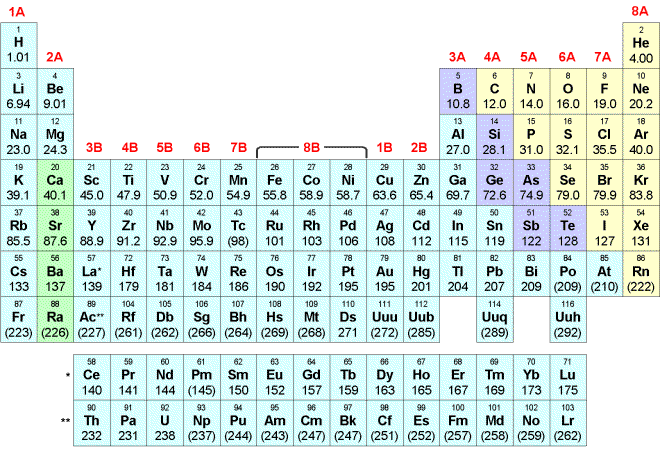
PERIODIC TABLE OF ELEMENTS
Select one answer for each question:
1. A piece of iron is heated from 25°C to 115°C. What is the temperature change of the iron in degrees Fahrenheit?
| A) | -50°F | |
| B) | 194°F | |
| C) | -130°F | |
| D) | 162°F | |
| E) | 50°F | |
2. How many picometers are there in one angstrom (Å)?
| A) | 1000 | |
| B) | 100 | |
| C) | 10 | |
| D) | 1 | |
| E) | 0.1 | |
3. What is the concentration of KNO3 after 40.0 mL of water is added to 160.0 mL of 0.128 M KNO3?
| A) | 0.116 M | |
| B) | 0.128 M | |
| C) | 0.102 M | |
| D) | 0.092 M | |
| E) | 0.142 M | |
4. What volume of 0.125 M barium hydroxide will exactly neutralize 200 mL of 0.160 M nitric acid?
| A) | 128 mL | |
| B) | 64 mL | |
| C) | 256 mL | |
| D) | 96 mL | |
| E) | 192 mL | |
5. The systematic name of the neutral compound ClO4 is:
| A) | chlorine(VIII) oxide | |
| B) | perchlorate | |
| C) | tetraoxygen chloride | |
| D) | chloric oxide | |
| E) | chlorine tetraoxide | |
6. Give the sum of the coefficients when the combustion equation is balanced with lowest integers:
__C6H14 + __
O2 ![]() __ CO2
+ __ H2O
__ CO2
+ __ H2O
| A) | 41 | |
| B) | 57 | |
| C) | 33 | |
| D) | 47 | |
| E) | 39 | |
7. The total number of ions released when 1.62 moles of calcium chloride dissolve completely in water is:
| A) | 1.95 ´ 1024 | |
| B) | 4.88 ´ 1024 | |
| C) | 2.93 ´ 1024 | |
| D) | 3.21 ´ 1024 | |
| E) | 1.46 ´ 1024 | |
8.
Two successive chemical reactions A ![]() B and B
B and B ![]() C have yields of 25% and 64%, respectively.
The yield for the overall conversion of A
C have yields of 25% and 64%, respectively.
The yield for the overall conversion of A ![]() C is:
C is:
| A) | 39% | |
| B) | 16% | |
| C) | 89% | |
| D) | 25% | |
| E) | 21% | |
9. Oxidation-reduction reactions involve transfer of
| A) | electrons from the reducing agent to the oxidizing agent | |
| B) | protons from the oxidizing agent to the reducing agent | |
| C) | electrons from the oxidizing agent to the reducing agent | |
| D) | protons from the reducing agent to the oxidizing agent | |
10. What is the volume of 1.25 moles of aluminum carbide (density = 2.36 g/mL)?
| A) | 47.8 mL | |
| B) | 76.3 mL | |
| C) | 20.7 mL | |
| D) | 55.6 mL | |
| E) | 69.9 mL | |
11. Give the moles of electrons exchanged in the balanced equation
As4S6
+ 9O2 ![]() As4O6 +
6SO2
As4O6 +
6SO2
| This type of redox problem will not be on the CH221 final | ||
| A) | 12 | |
| B) | 0 | |
| C) | 48 | |
| D) | 36 | |
| E) | 24 | |
12. Find the oxidation number of nitrogen in a compound NxOy if 7.143 g of the compound contains 2.597 g of oxygen.
| A) | 1 | |
| B) | 4 | |
| C) | 3 | |
| D) | 2 | |
| E) | 5 | |
13. Use the following reaction data
Fe2O3 + 3CO ![]() 2Fe + 3CO2
, DH = -26.8 kJ
2Fe + 3CO2
, DH = -26.8 kJ
FeO + CO ![]() Fe + CO2 ,
DH
= -16.5 kJ
Fe + CO2 ,
DH
= -16.5 kJ
to find the enthalpy change for the reaction
Fe2O3
+ CO ![]() 2FeO +
CO2
2FeO +
CO2
| A) | 27.7 kJ | |
| B) | 10.3 kJ | |
| C) | 6.2 kJ | |
| D) | - 43.3 kJ | |
| E) | -10.3 kJ | |
14. Which of these samples of ionic compounds releases the largest number of ions when placed in water?
| A) | 0.3 mole of Al(OH)3 | |
| B) | 0.2 mole of NaCl | |
| C) | 0.2 mole of PbBr2 | |
| D) | 0.1 mole of (NH4)2CO3 | |
| E) | 0.4 mole of CaSO4 | |
15. How many moles of electrons can be transferred in a fuel cell based on the equation
2H2 + O2
![]() 2H2O
2H2O
with 12 g of hydrogen and 72 g of oxygen.
(HINT: this involves a limiting reactant.)
| this type of redox problem will not be on the CH221 final | ||
| A) | 18 | |
| B) | 6 | |
| C) | 15 | |
| D) | 9 | |
| E) | 12 | |
16. Identify the oxidizing agent in the equation:
H3IO62- + HSnO2-
+ H2O
![]() IO3- +
Sn(OH) 62-
IO3- +
Sn(OH) 62-
| A) | HSnO2- | ||
| B) | Sn(OH) 62- | ||
| C) | H2O | ||
| D) | IO3- | ||
| E) | H3IO62- | ||
17. Which mixture of solutions does not produce a solid precipitate?
| A) | potassium iodide and lead(II) nitrate | |
| B) | silver(I) nitrate and sodium chloride | |
| C) | calcium nitrate and sodium sulfide | |
| D) | copper(II) sulfate and ammonium carbonate | |
| E) | sodium hydroxide and cobalt(II) chloride | |
18. What is the wavelength of the electromagnetic radiation from a cell phone that operates at 8.50 ´ 108 s-1 ?
| A) | 35.3 cm | |
| B) | 25.4 cm | |
| C) | 15.6 cm | |
| D) | 52.9 cm | |
| E) | 43.1 cm | |
19. What is the principal quantum number of the occupied orbital in the ground state of the hydrogen atom?
| A) | 2 | |
| B) | 1 | |
| C) | 0 | |
| D) | -1 | |
| E) | -2 | |
20. What is the angular momentum quantum number for a 3p orbital?
| A) | 1 | |
| B) | 3 | |
| C) | 2 | |
| D) | 0 | |
| E) | 4 | |
21. According to ________ a particle of mass m and velocity v has a wavelength l = h/mv.
| A) | Planck | |
| B) | Rydberg | |
| C) | Bohr | |
| D) | Einstein | |
| E) | de Broglie | |
22. The allowed values of ml when n = 3 and l = 2 in the hydrogen atom are:
| A) | 0, 1, 2, 3 | |
| B) | 3, 2, 1, 0, -1, -2, -3 | |
| C) | 0, 1, 2 | |
| D) | 2, 1, 0, -1, -2 | |
| E) | 1, 0, -1 | |
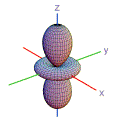
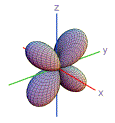
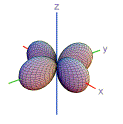
23. Which of these shapes are consistent with atomic orbitals that have n = 2?
| 1) | 2) | 3) | 4) | 5) | |
 |
 |
 |
 |
 |
| A) | 3, 5 | |
| B) | 1, 2, 4 | |
| C) | 1, 3, 5 | |
| D) | 2, 3, 4, 5 | |
| E) | 1, 5 | |
24. What
is the radius of a hydrogen-like ion F8+ with n = 2 in the Bohr model?
| A) | 30.2 pm | |
| B) | 23.5 pm | |
| C) | 34.6 pm | |
| D) | 18.7 pm | |
| E) | 26.5 pm | |
25. Which of these transitions in the hydrogen atom emits radiation at the longest wavelength?
| A) | 4 |
|
| B) | 2
|
|
| C) | 5 |
|
| D) | 3 |
|
| E) | 100
|
|
26. Which atom has the electron configuration 1s2 2s2 2p6 3s2 3p2 ?
| A) | oxygen | |
| B) | aluminum | |
| C) | sulfur | |
| D) | phosphorus | |
| E) | silicon | |
27. The maximum number of 4d electrons is:
| A) | 12 | |
| B) | 18 | |
| C) | 14 | |
| D) | 10 | |
| E) | 16 | |
28. What is the highest value of n in the electron configuration for Pb?
| A) | 6 | |
| B) | 3 | |
| C) | 7 | |
| D) | 5 | |
| E) | 4 | |
29. How many “p” electrons are there in Br?
| A) | 18 | |
| B) | 16 | |
| C) | 17 | |
| D) | 21 | |
| E) | 15 | |
30. In which atom does the electron configuration in the highest unfilled subshell look like this?
|
|
|||
| A) | Si | ||
| B) | S | ||
| C) | Sc | ||
| D) | Sn | ||
| E) | Sb | ||
31. What is the energy of one mole of photons whose wavelength is 319 nm?
| A) | 288 kJ | |
| B) | 375 kJ | |
| C) | 623 kJ | |
| D) | 324 kJ | |
| E) | 415 kJ | |
32. What mass of oxygen is consumed in the combustion of 63.4 g of acetaldehyde by the equation
2CH3CHO(l) + 5O2(g)
![]() 4CO2(g) +
4H2O(l)
4CO2(g) +
4H2O(l)
| A) | 110 g | |
| B) | 57.6 g | |
| C) | 233 g | |
| D) | 115 g | |
| E) | 221 g | |
33. How much heat is produced in the preceding Question 32?
| A) | 1609 kJ | ||
| B) | 1736 kJ | ||
| C) | 1681 kJ | ||
| D) | 1592 kJ | ||
| E) | 1719 kJ | ||
