
Select the best single answer for each question:
1. Which is a strong electrolyte?
1. HCl 2. KOH 3. HNO2
| A) | 1 only | |
| B) | 2 only | |
| C) | 3 only | |
| D) | 1 and 2 only | |
| E) | 1, 2 and 3 only | |
2. The total moles of ions released when 3 moles of Al2(SO4)3 dissolve completely in water is
| A) | 15 | |
| B) | 12 | |
| C) | 9 | |
| D) | 18 | |
| E) | 21 | |
3. When HNO3 reacts with KOH in aqueous solution the net ionic equation is
| A) | K+(aq)
+ NO3-
(aq) |
|
| B) | OH-(aq)
+ H+(aq) |
|
| C) | H+(aq) + KOH(aq) |
|
| D) | OH-(aq)
+ HNO3(aq) |
|
4. It
takes 10,600 kJ each day to maintain a body weight of 80 kg (about 175 lb).
How
many kilocalories is this each day?
| A) | 1275 | |
| B) | 4435 | |
| C) | 2190 | |
| D) | 3548 | |
| E) | 2533 | |
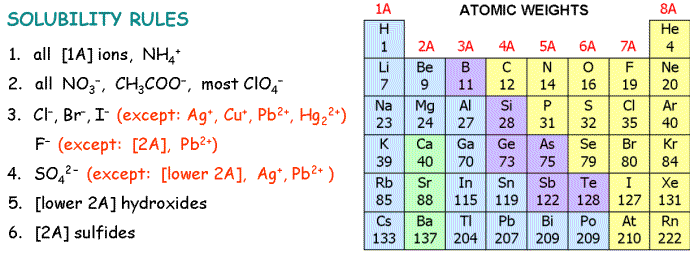
5. Which of these compounds is most soluble in water?
| A) | CuS | |
| B) | PbSO4 | |
| C) | ZnCl2 | |
| D) | Ag2CO3 | |
6. Oxidation-reduction reactions involve transfer of
| A) | electrons from the reducing agent to the oxidizing agent | |
| B) | protons from the oxidizing agent to the reducing agent | |
| C) | electrons from the oxidizing agent to the reducing agent | |
| D) | protons from the reducing agent to the oxidizing agent | |
7. How many mL of 0.200 M NaOH neutralize 50.0 mL of 0.760 M HNO3 ?
| A) | 124 | |
| B) | 190 | |
| C) | 232 | |
| D) | 168 | |
| E) | 215 |
8. The oxidation number of vanadium in HV6O173- is
| A) | 9 | |
| B) | 8 | |
| C) | 4 | |
| D) | 5 | |
| E) | 6 | |
9. Which is NOT an oxidation-reduction reaction?
| A) | 4
AgNO3 + SnCl4 |
|
| B) | S8
+ 24 F2 |
|
| C) | ClO-
+
NO2-
|
|
| D) | 2
Cr + 3 Sn2+ |
|
10. Find the oxidizing agent in the equation
6 Ag+ + 3 S + 2 NO + 4 H2O
![]() 3 Ag2S + 8 H+ + 2 NO3-
3 Ag2S + 8 H+ + 2 NO3-
| A) | NO | |
| B) | H2O | |
| C) | Ag+ | |
| D) | S | |
11.
Use the data to find the enthalpy change for the reaction Br + H2
![]() HBr + H
HBr + H
H2 ![]() H + H ,
DH
= 436 kJ
H + H ,
DH
= 436 kJ
Br2 ![]() Br + Br,
DH
= 193 kJ
Br + Br,
DH
= 193 kJ
HBr ![]() H + Br,
DH
= 366 kJ
H + Br,
DH
= 366 kJ
| A) | -243 kJ | |
| B) | 70 kJ | |
| C) | 173 kJ | |
| D) | 243 kJ | |
| E) | -173 kJ |
12.
A chemical process at constant
pressure transfers 200 kJ of heat to the surroundings.
The enthalpy change of the system is best described as:
| A) | DH = 200 kJ (endothermic) | |
| B) | DH = 200 kJ (exothermic) | |
| C) | DH = -200 kJ (exothermic) | |
| D) | DH = -200 kJ (endothermic) | |
13. How much energy (J) is required to heat 151 kg of water (about 40 gal in a home water heater)
from 18.0°C to 50.0°C? The heat capacity of water is 4.184 J/g-°C.
| A) | 2.65 ´ 107 J | |
| B) | 1.52 ´ 107 J | |
| C) | 9.96 ´ 106 J | |
| D) | 2.02 ´ 107 J | |
| E) | 1.52 ´ 104 J | |
14. Which of these has the highest oxidation number for sulfur?
| A) | Na2S2O8 | |
| B) | H2S | |
| C) | SO2 | |
| D) | S8 | |
15. How many mL of 1.00 M HCl react with 0.0350 moles of calcium hydroxide by
Ca(OH)2 + 2 HCl ![]() CaCl2 + 2 H2O
CaCl2 + 2 H2O
| A) | 50.0 | |
| B) | 35.0 | |
| C) | 60.0 | |
| D) | 25.0 | |
| E) | 70.0 | |
16. Use the heats of formation to find DH° for the reaction
|
N2O(g) + 3H2(g) |
DH°f |
|||||
| A) | -153 kJ | N2O(g) | 82 kJ | |||
| B) | -273 kJ | N2H4(l) | 51 kJ | |||
| C) | -133 kJ | H2O(l) | -286 kJ | |||
| D) | -204 kJ | H2O(g) | -242 kJ | |||
| E) | -317 kJ | |||||
17. Mixing solutions of barium hydroxide and magnesium sulfate yields
| A) | aqueous barium sulfate and solid magnesium hydroxide | |
| B) | aqueous barium sulfate and aqueous magnesium hydroxide | |
| C) | solid barium sulfate and aqueous magnesium hydroxide | |
| D) | solid barium sulfate and solid magnesium hydroxide | |
18. Find the number of electrons transferred in the balanced oxidation-reduction reaction
2 C6H6 + 15 O2
![]() 12 CO2 +
6 H2O
12 CO2 +
6 H2O
| This type of problem will NOT be covered on the exam. | ||||
| A) | 24 | |||
| B) | 40 | |||
| C) | 48 | |||
| D) | 36 | |||
| E) | 60 | |||
19.
56.5 mL of 0.100M
NaOH solution neutralizes 260 mg of an unknown acid HA.
Which of the following acids has a
molecular weight closest to the unknown?
| A) | HOCl | |
| B) | HCN | |
| C) | HCOOH | |
| D) | HF | |
| E) | CH3COOH | |
20. The standard heat of formation of H2O(g) is the enthalpy change for the reaction
| A) | 2H(g) + O(g) |
|||
| B) | 2H2(g)
+ O2(g) |
|||
| C) | H2O(g) |
|||
| D) | H2(g)
+ 0.5 O2(g) |
|||
| E) | H2O(l)
|
|||
![]()